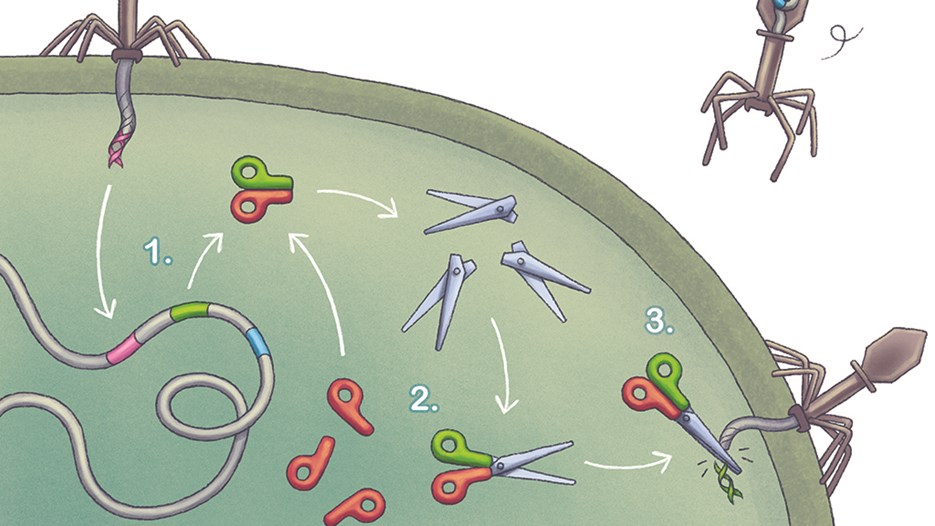
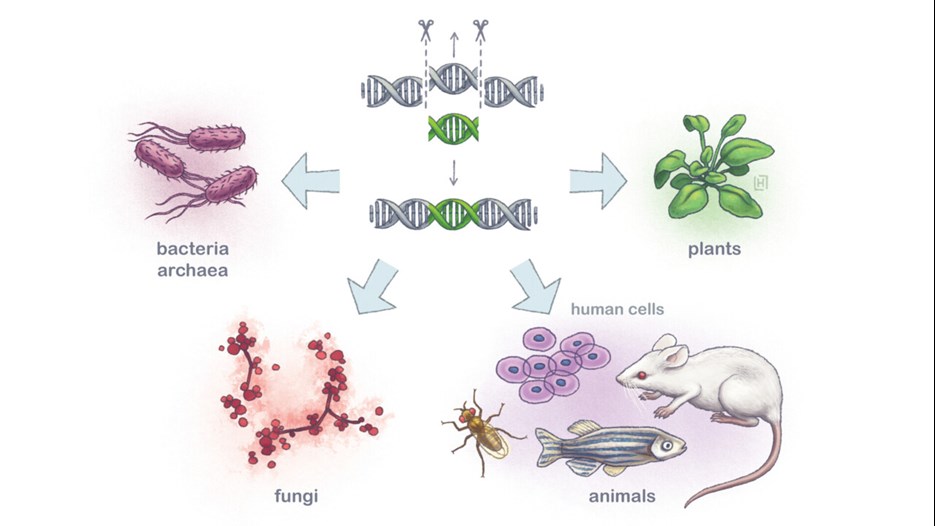
Emmanuelle Charpentier
Born: In 1968 in Juvisy-sur-Orge, 20 km south-east of Paris, France.
Position: Scientific, Managing and Founding Director at the Max Planck Unit for the Science of Pathogens, Berlin, Germany. She is also Honorary Professor at the Humboldt-Universität zu Berlin, Germany. Previously group leader at the Helmholtz Centre for Infection Research and Braunschweig/Hannover Medical School in Germany, and visiting professor and group leader at Umeå University. Appointed honorary doctor at Umeå University in 2017.
Leisure: “I have been very busy with work in recent years and even more as a result of all the attention surrounding CRISPR-Cas9, but I really try to keep up with other interests too, such as sporting activities. I am very much interested in culture, art and design. I can at least find the time to listen to music while working, walking and thinking, and I enjoy listening to debates by philosophers and sociologists that question the world and our society. This is where I find my energy and balance.”
Best mode of transport: “Bike! I cycle wherever I am – Paris, New York, Vienna, Umeå, Braunschweig – and currently on a daily basis in Berlin.”
About moving to Umeå: “Perhaps some people wondered how a French city girl would get on here, but I settled in very well. It’s an international environment with researchers and students from around the world, which is something you notice when you go into the city and see what it has to offer culturally. I have also been taken by the nature here, and the snow and dry cold are more enjoyable than the rainy winters in Vienna.”