About the publication:
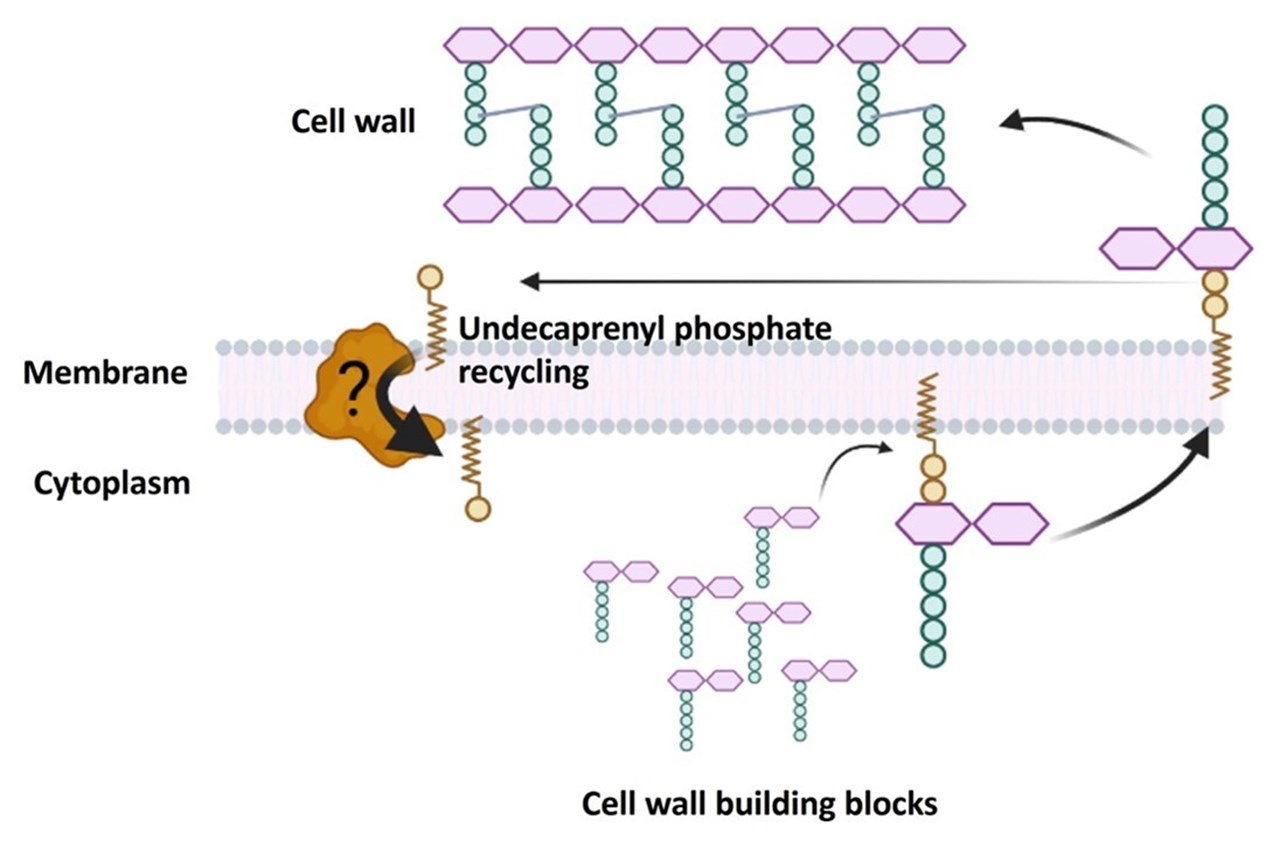
Brandon Sit, Veerasak Srisuknimit, Emilio Bueno, Franz G. Zingl, Karthik Hullahalli, Felipe Cava, Matthew K. Waldor (2022): Undecaprenyl phosphate translocases confer conditional microbial fitness. Nature (2022). https://doi.org/10.1038/s41586-022-05569-1