
Image: Mattias Pettersson

Image: Mattias Pettersson
Research group We study the role of bacterial genotoxins in modulation of intestinal mucosa homeostasis.
Teresa Frisan Lab has a long-standing interest in understanding the effects on eukaryotic cells and the role on acute and chronic bacterial infections of a relative novel family of bacterial toxins, defines as genotoxins, since they induce DNA damage in the host cells.
At present, only three bacterial genotoxins have been identified: colibactin produced by Escherichia coli (including commensal and probiotic strains), Klebsiella pneumoniae, Enterobacter aerogenes, and Citrobacter koseri; the cytolethal distending toxin (CDT) family produced by several Gram-negative pathogens; the typhoid toxin produced by Salmonella enterica serovar Typhi and some other nontyphoidal Salmonella (NTS) serotypes.
The initial focus of the lab was to understand the mode of action of the first discovered member of this family: CDT, which was still not characterized. We have shown that exposure to high toxin doses activates the classical DNA damage response (DDR), orchestrated by DDR sensor kinase Ataxia Telengiectasia Mutated (ATM) kinase leading to activation of checkpoint responses that block cell proliferation and activate DNA repair mechanisms. If the DNA damage is beyond repair, most of the mammalian cell types tested undergo senescence, while T and B lymphocytes are more susceptible to die by apoptosis. Conversely, exposure to low toxin doses have shown to promote classical signs of carcinogenesis, such as cell survival and acquisition of genomic instability.
There are several puzzling questions that still need to be addressed in this field, such as: why bacteria have acquired these unusual effectors that target the mammalian DNA, and differently from other well-characterized bacterial toxins, do not cause an immediate cell death in most of the host cells, but promote senescence, characterised by an active secretory phenotype? Why intestinal commensal bacteria and probiotics also produce these effectors? Is the role of the genotoxins different when produced by pathogenic bacteria? Can these toxins exert carcinogenic effects in chronic infections and if yes in which conditions?
To answer these questions, we have developed an in vivo model, to assess the role of the typhoid toxin produced by S. Typhi. We have chosen this bacterium because is the only genotoxin-producing bacterium that can establish chronic infections and is associated with increased risk of tumor development in humans. However, this poses a challenge since S. Typhi is an exclusive human pathogen. Therefore, we have constructed several S. Typhimurium strains expressing either a functional (Figure 1) or non-functional typhoid toxin and causing a systemic typhoid-like infection in immunocompetent mice.
PhotoRiccardo Guidi
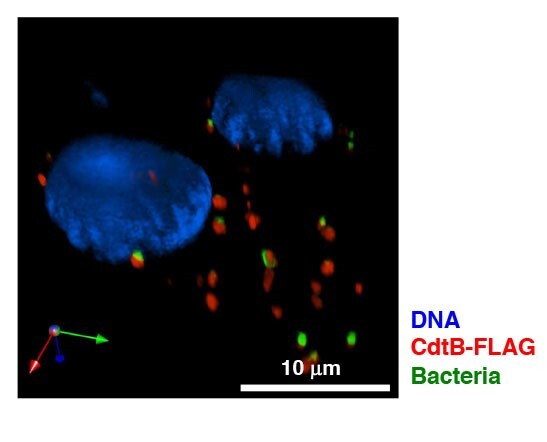
Figure 1. Detection of the DNA damaging CdtB subunit (red) of the typhoid toxin by immunofluorescence analysis in human intestinal epithelial Caco-2 cells infected for 24h with a GFP-labeled S. Typhimurium strain MC1-TT (green). Nuclei are counterstained with DAPI (blue).
Unexpectedly, infection with S. enterica expressing the typhoid toxin does not cause acute cell death but plays a key role in reprogramming the host immune response towards an anti-inflammatory status (Figure 2) and favors the establishment of a persistent infection. This effect on the host immune response is tissue specific, since it is observed in colon, but not in liver and spleen. The results indicate that this unusual bacterial effector is not a classical toxin, but acts as immunomodulator, highlighting a complex and tissue specific crosstalk between two very conserved stress responses: the immune response and the DNA damage response (DDR).
PhotoIoannis S. Pateras
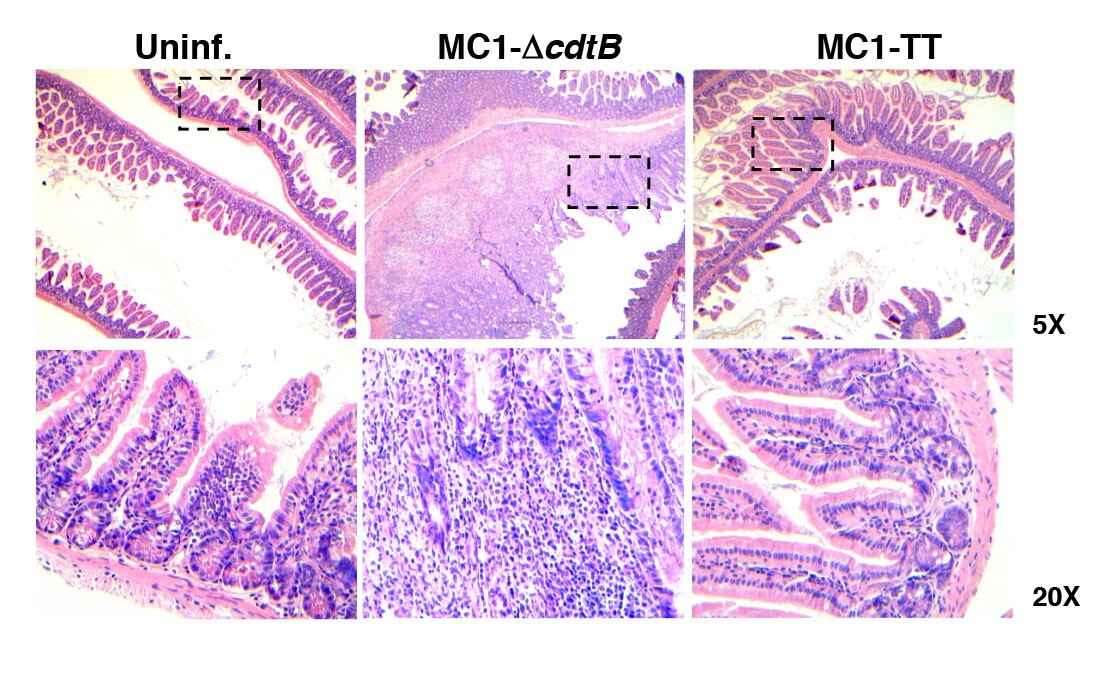
Figure 2. Haematoxylin and eosin staining of the intestine of uninfected mice or mice infected for 10 days with the control (MC1-ΔcdtB) or genotoxigenic MC1 (MC1-TT) strains. The dashed squares indicate the area that is enlarged in the lower panel. Note the prominent inflammatory response induced by the control strain, which is absent in mice infected with the bacterium expressing the typhoid toxin.
The next step is to understand how activation of the DDR modulates the host immune response, and whether the final outcome is different in healthy subjects and in individuals who are susceptible to chronic inflammation or cancer. To address this issue, we are using a combination of in vivo and 3D human organotypic models (Figure 3).
PhotoOceane C.B. Martin
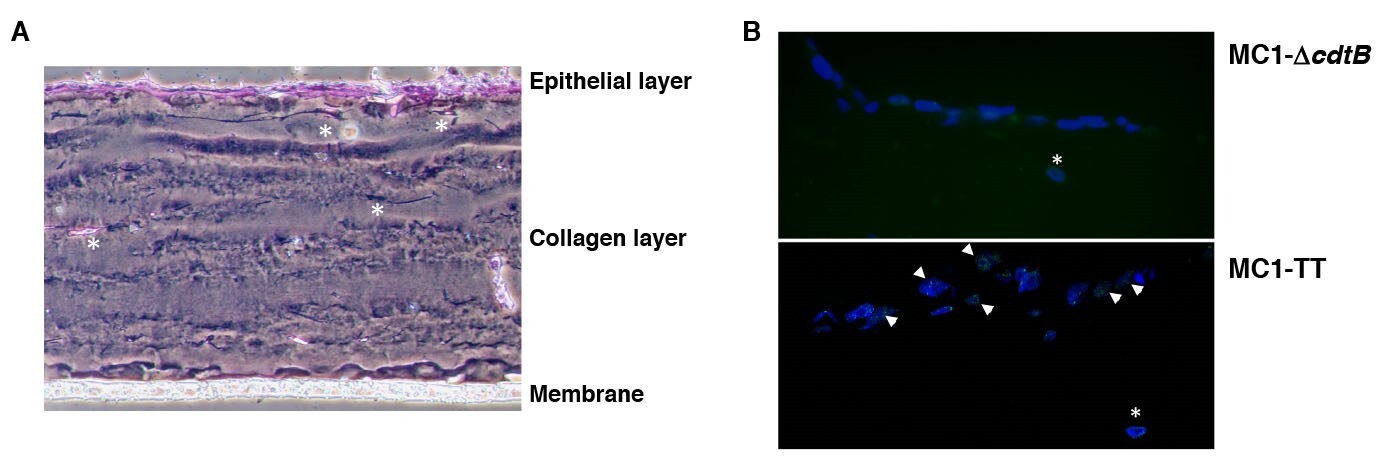
Figure 3. Organotypic 3D models. A. Phase contrast micrograph of colonic epithelial cells, grown in 3D culture and stained with hematoxylin and eosin. B. Colonic epithelial cells infected with the control (MC1-ΔcdtB) or genotoxigenic MC1 (MC1-TT) strains for 24h. Activation of the DDR was assessed by immunofluorescence analysis, using antibodies specific for 53BP1 (green). Nuclei were counterstained with DAPI (blue). The arrows indicate cell positive for the formation of 53BP1 foci at the site of the damaged DNA. The white asterisks indicate the presence of colonic fibroblasts embedded in the collagen matrix.
Our research on cancer focus on Salmonella bacterial genotoxins, which cause damage in cells, and in turn infection, inflammation and cancer.
Epidemiological evidence linked chronic bacterial infections or altered intestinal microbiota (dysbiosis) with chronic inflammation and cancer. However, the molecular mechanisms that promote tumour initiation/progression, determine dysbiosis, and whether this is the cause or the consequence of the inflammatory/cancerous lesions are not known.
In this complex context, we study the contribution of bacterial genotoxins, which cause DNA damage and cellular senescence in the host cells, in the establishment of chronic infections and the alteration of the gut-hepatobiliary tract homeostasis. Colonization with genotoxin-producing bacteria can contribute to tumour development under specific circumstances. However, the biological relevance of these toxins remains to be determined, since it is unlikely that their primary function is to promote cancer in the host. To address this issue, we developed a model based on Salmonella Typhimurium, expressing the genotoxin known as typhoid toxin. It is noteworthy that Salmonella is the only genotoxin-producing bacterium associated with cancer in humans.
Specifically, we are interested in understanding the role of the tissue microenvironment, toxin-induced cellular senescence and immunological landscapes in healthy subjects or in conditions prone to inflammatory diseases or cancer in defining the pro-tumorigenic outcome of the bacterial infection. Progress in this field has been hampered by the complexity of the systems, which includes many variables such as the tissue specific microbial ecology and the host immune response. To solve this complex interplay, we developed a in vivo model and are setting up an immune competent 3D organotypic tissue culture system, which will allow us to clarify the molecular mechanisms of bacteria-associated carcinogenesis. This research may result in specific therapeutic protocols aimed at an early, rapid and selective eradication of bacterial populations in patients, thus preventing of the initial steps of tumour development, promoting health and reducing the cost of cancer therapy.

Javier Avila-Cariño, Teresa Frisan, Anna Bergonzini and Maria Lopez Chiloeches.
PhotoAnna BergonziniTeresa Frisan, PhD
Professor
e-mail: teresa.frisan@umu.se
Javier Avila-Cariño, PhD
Senior researcher
e-mail: Javier.Avila-Carino@ki.se
Anna Bergonzini
PhD student
e-mail: anna.bergonzini@umu.se
Maria Lopez Chiloeches, Post Doc
e-mail: maria.chiloeches@umu.se
Ioannis Pateras, Guest researcher
Publications of interest
Océane C.B. Martin, Anna Bergonzini, Maria Lopez Chiloeches, Eleni Paparouna, Deborah Butter, Sofia D.P. Theodorou, Maria M. Haykal, Elisa Boutet-Robinet, Toma Tebaldi, Andrew Wakeham, Mikael Rhen, Vassilis G. Gorgoulis, Tak Mak, Ioannis S. Pateras, and Teresa Frisan. Influence of the microenvironment on the modulation of the host response by the typhoid toxin (accepted Cell Reports).
Papalampros A, Vailas M, Ntostoglou K, Chiloeches ML, Sakellariou S, Chouliari NV, Samaras MG, Veltsista PD, Theodorou SDP, Margetis AT, Bergonzini A, Karydakis L, Hasemaki N, Havaki S, Moustakas II, Chatzigeorgiou A, Karamitros T, Patsea E, Kittas C, Lazaris AC, Felekouras E, Gorgoulis VG, Frisan T*, Pateras IS*. (2020). Unique Spatial Immune Profiling in Pancreatic Ductal Adenocarcinoma with Enrichment of Exhausted and Senescent T Cells and Diffused CD47-SIRPα Expression. Cancers 12, 1825
* shared senior authorship
Martin OCB, Bergonzini A, Federica D’Amico F, Chen P, Shay JW, Dupuy J, Svensson M, Masucci MG, Frisan T. (2019). Infection with genotoxin-producing Salmonella enterica synergises with loss of the tumor suppressor APC in promoting genomic instability via the PI3K pathway in colonic epithelial cells. Cell Micro 21, e13099.
Frisan T, Nagy N, Chioureas D, Terol M, Grasso F, Masucci MG (2018). A bacterial genotoxin causes virus reactivation and genomic instability in Epstein-Barr virus infected epithelial cells pointing to a role of co-infection in viral oncogenesis. Int J Cancer 144(1): 98-109.
Seiwert N., Neitzel C., Stroh S., Frisan T., Audebert, M, Toulany M., Kaina B., and Fahrer J. (2017). AKT2 suppresses pro-survival autophagy triggered by DNA double-strand breaks in colorectal cancer cells: Cell Death and Disease 8, e3019.
Del Bel Belluz L, Guidi R, Pateras IS, Levi L, Mihaljevic B, Rouf SF, Wrande M, Candela M, Turroni S, Nastasi C, Consolandi C, Peano C, Tebaldi T, Viero G, Gorgoulis VG, Krejsgaard T, Rhen M, Frisan T. (2016). The Typhoid Toxin Promotes Host Survival and the Establishment of a Persistent Asymptomatic Infection. PLoS Pathog. 12, e1005528.
This paper was highlighted in Nature (2016), 532, 521: "Salmonella lives on thanks to toxin", and has attracted scientific media attention (http://www.eurekalert.org/pub_releases/2016-04/p-tti033116.php; https://www.sciencenews.org/article/typhoid-toxin-aids-survival-mice?tgt=nr).
Levi L., Toyooka T., Patarroyo M., Frisan T. (2015) Bacterial genotoxins promote inside-out integrin activation, formation of focal adhesion complexes and cell spreading. PLosOne PLoS One. 13, e0124119.
Fahrer J., Huelsenbeck J., Jaurich H., Dörsam B., Frisan T., Eich M., Roos W.P., Bernd Kainaa, Fritz G. (2014). Cytolethal distending toxin (CDT) is a radiomimetic agent and induces persistent levels of DNA double-strand breaks in human fibroblasts. DNA repair 18, 31-43.
Guidi R, Levi L, Rouf SF, Puiac S, Rhen M, Frisan T. (2013). Salmonella enterica delivers its genotoxin through outer membrane vesicles secreted from infected cells. Cell. Micro. 15, 2034-2050.
Guidi R., Guerra L., Levi L., Stenerlöw B., Fox J.G., Josenhans C., Masucci M.G., Frisan T. (2013). Chronic exposure to the cytolethal distending toxins of Gram-negative bacteria promotes genomic instability and altered DNA damage response. Cell. Micro. 15, 98-113.
Frisan T., Coppotelli G., Dryselius R., Masucci M.G. (2012). Ubiquitin C-terminal hydrolase-L1 interacts with adhesion complexes and promotes cell migration, survival, and anchorage independent growth. FASEB J 26, 5060-70.
Guerra L., Guidi R., Slot I., Callegari S., Sompallae R., Carr H.S., Pickett C.L., Åström S., Eisele F., Wolf D., Frost J.A., Sjögren C., Masucci M.G., Frisan T. (2011) DNA damage triggers FEN1-dependent cytoskeleton remodeling and cell survival. J of Cell Science, 124, 2735-2742.
Guerra L., Albihn A., Tronnersjö S., Yan Q., Guidi R., Stenerlöw B, Sterzenbach T., Josenhans C., Fox J.G., Schauer D.B., Thelestam M., Larsson LG, Henriksson M. and Frisan T. (2010). Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage. PLoS One, 5:e8924.

'EC' Postdoc Baptiste marvels at how molecular mechanisms shape cell destiny and physiology.
Umeå researchers have discovered that bacterial toxins can act as modulators of the host immune response.