Image: Margareta Norberg

Image: Margareta Norberg
Research project VIsualiZation of asymptomatic Atherosclerotic disease for optimum cardiovascular prevention ─ a pragmatic randomized controlled trial nested in the Västerbotten Intervention Program. Prevention of cardiovascular disease (CVD) often fails due to low adherence among physicians and individuals to prevention guidelines. Therefore, new and alternative approaches are needed. VIPVIZA evaluates the potential of visualization of silent atherosclerosis as the basis for prevention.
VIPVIZA inform patients and their physicians with graphs and pictures about subclinical atherosclerosis aiming at improved CVD risk perception and adherence to CVD prevention guidelines. After one year, a reduction of Framingham Risk Score was shown, in contrast to an increase in the control group, where no ultrasound results were given. The difference between groups was similar after three years. The CVD risk atherosclerosis and CVD morbidity and mortality up to 10 years, and the intervention impact on biomarkers and social and psychological determinants of behavioral change will be evaluated
VIPVIZA is funded by Västerbotten County Council (Central ALF, ALFVLL-298001, -643391, Spjutspets VLL-583721), the Swedish Research Council (Dnr 521-2013-2708, 2016-01891), the Heart and Lung Foundation (Dnr 20150369, 20170481), the Swedish Society of Medicine (SLS-405351, -503111), and SKANDIA Risk/Health.
In addition to major grants, VIPVIZA was funded by the Heart Foundation of Northern Sweden, STROKE-the National Association, the Foundation for Stroke Research in Northern Sweden, The Swedish Insurance Society, Visare Norr (the four Northern Regions) (465621, 561591, 741711, 931135), Foundations managed by the Faculty of Medicine Umeå University, and The Swedish and the Västerbotten Heart and Lung Associations.
An unconditional donation was received from Carl Bennet Ltd, Sweden.


The main objective of this project is to contribute to improved primary prevention of cardiovascular disease above conventional CVD risk screening and prevention through the provision of a visual image and pictorial report of atherosclerosis while still asymptomatic. The image and report are seen and discussed by both the physician and the patient in order to improve the physician’s assessment of patients’ CVD risk and guideline adherence, as well as to improve the patients’ risk perception and enhance his/her motivation for performance of prevention measures. The specific objectives include: 1.To assess the prevalence of asymptomatic atherosclerotic disease in men and women through identification of carotid plaques and measurement of carotid intima-media thickness (CIMT), and to relate plaques and CIMT to clinically estimated CVD risk factors and risk scores 2.To explore the impact of pictorial representations of atherosclerosis on physicians´ adherence to prevention guidelines, and on individuals’ quality of life, preventive measures, risk factor control and progress of atherosclerotic disease over the course of three and six years, as well as on premature CVD morbidity and mortality over the course of 10 years 3.To evaluate how individuals’ social, psychological, cognitive characteristics relate to atherosclerosis and CVD risk at baseline and progression of CVD risk and atherosclerosis 4.To investigate biomarkers in relation to CIMT and plaques at baseline, changes in conventional CVD risk markers and lifestyle, and progression of atherosclerosis.
Primary prevention of CVD often fails due to poor adherence among practitioners and patients to evidence-based prevention guidelines on effective modification of risk factors by lifestyle change and pharmacological treatment. Contributory factors include poor communication about the CVD risk by the physician and inaccurate risk perception among patients. The risk message is usually communicated verbally or numerically, while potentially more effective visual tools are seldom used. For the clinical assessment of CVD risk Framingham risk score (FRS) and the European systematic coronary risk evaluation (SCORE) are most widely used. However, evidence that their use translates into reduced CVD morbidity and mortality is scarce. These risk scores focus on high-risk individuals, despite 60-70% of all CVD events occurring among individuals at low or intermediate risk for CVD. They might also be too abstract to lead to accurate risk perception and to motivate for preventive actions; information alone seldom results in rational behavior modification. VIPVIZA takes a different approach from current practice for the prevention of CVD. Instead of being based solely on indirect risk factors, this project evaluates the atherosclerotic disease itself while it still is silent, providing improved assessment, communication and perception of the CVD risk and hence greater motivation for prevention. This is achieved with ultrasonography of medium sized arteries with assessment of Carotid Intima media thickness (CIMT) and existing atherosclerotic plaques.
The study is a pragmatic randomized open-label controlled trial with blinded evaluators (PROBE) with single-arm cross-over of the control-group at the 3-year follow-up. It is registered at https://clinicaltrials.gov/ct2/show/NCT01849575, where a detailed study protocol is presented. VIPVIZA is integrated in and added to the ordinary Västerbotten Intervention Programme (VIP). Individuals with at least one clinical CVD risk factor were invited to the VIPVIZA trial when they participated in VIP (n=4177), resulting in inclusion of 3532 participants. Baseline visits with ultrasound examinations were carried out from April 2013 to June 2016. Participants were consecutively and randomly allocated to two groups (intervention and control group) using a computer-generated randomization list. The ultrasound examinations in VIPVIZA at baseline as well as after three years were performed at the hospitals in the three cities/towns (Umeå, Skellefteå, Lycksele), and in remote rural areas at primary health care centres. Risk factor measurements and questionnaires at follow-up after one and three years were carried out for participants living in Umeå at the Clinical Research Centre at Umeå University Hospital, and for participants in the rest of the county at their local primary health care center, and risk factor results were given to all participants and their GPs. The 6.5-year follow-up examinations are using the same methodology and routines, they were commenced in December 2019 and are expected to be completed December 2022. The progress of the study is according to the plan. Both groups are managed according to clinical guidelines for CVD prevention within primary care (not by the study team).
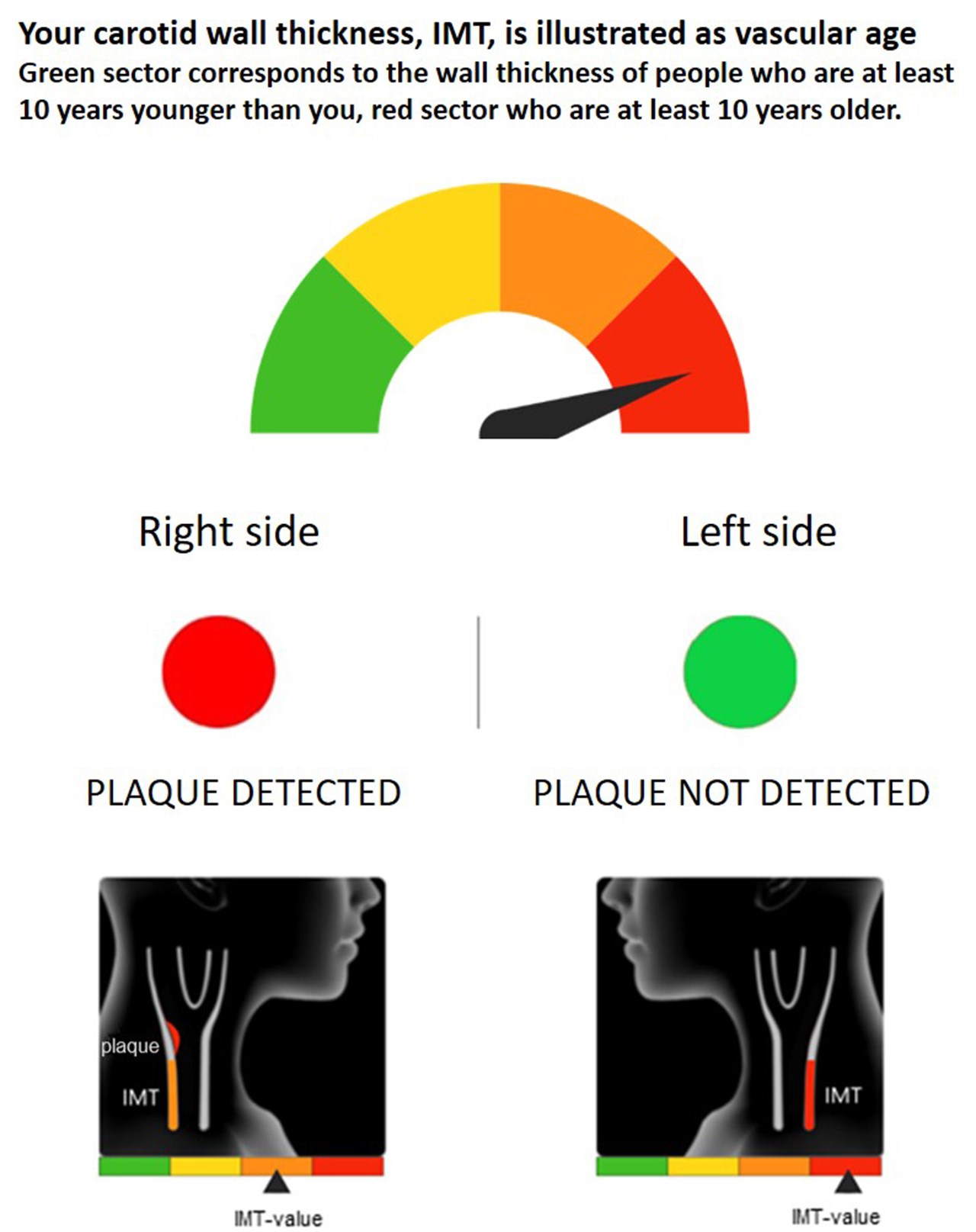
VIPVIZA ultrasound result image
ImagePictorial representation of the carotid ultrasound results was sent to each participant in the intervention group and to their primary care physician 1-2 weeks after the carotid ultrasonography. Atherosclerosis was presented as vascular age, with a gauge ranging from green through yellow and orange to red. This illustrated the individual’s biological, compared to chronological, age. A red or a green circle for each side, like a traffic light, showed if any plaque was detected or not, respectively. A stylized picture of the individuals’ ultrasound image was also provided. Brief written information about atherosclerosis as a dynamic process that is modifiable by a healthy lifestyle and preventive pharmacological treatment, as well as an interpretation of the result and general advice on CVD prevention were included. After additionally2-4 weeks, participants received a follow-up phone call by a research nurse to reassure and give additional clarification and information as needed. The same pictorial information was repeated to participants in the intervention group after 6 months. No information about the ultrasound result was given to the control group participants and their physicians and was also not available within health care.
After the 3-year examinations, the ultrasound result was given to all study-participants and their physicians in primary care. Thus, a single arm-crossover was performed, where the intervention was provided for the second time to the intervention group, and for the first time to the control-group. The same examinations and procedures as at the 3-year examination were performed at the 6.5-year follow-up. Tus the intervention was provided for the third time to the intervention group and second time to the control group.
Time line
Baseline: April 2013-June 2016.
1-Year follow-up: May 2014-Nov 2017.
3-year follow-up: Aug 2916-June 2019.
6,5-year follow-up: Dec 2019 to be completed Feb 2023.
Hard outcomes after 10 years: Planned to be retrieved from registers at the earliest 2027, due to known delay until registers are updated (Total and CVD deaths, CVD morbidity and mortality, revascularizing procedures).
Clinical risk factors for cardiovascular disease: Measured at the baseline VIP health survey and at 1-, 3-, and 6.5-year follow-ups (blood pressure, lipids, and glucose, BMI and waist circumference).
Questionnaires: The VIP questionnaire covers health, socioeconomic situation, quality of life (RAND 36), lifestyle (physical activity, tobacco and alcohol consumption, diet), working conditions, social network. Validated psychometric instruments at baseline, 3- and 6,5-year follow-up included health literacy, coping strategies, an optimism-pessimism scale, self-efficacy, HADS and self-rated risk of CVD. Perceptions about preventive medication, Food-frequency-questionnaire as well as perceptions about the intervention and emotional and cognitive reactions to the intervention were added at the 3-year follow-up. Questionnaires on stress, personality, and on attitudes and norms regarding healthy lifestyle habits were added at the 6-year follow-up.
At the 6-5-year follow-up, also objective testing of physical activity with accelerometry, measurement of hand grip strength, and participants’ dental health (information from dental records and panorama x-ray images) were included.
Carotid ultrasound examinations are performed at baseline and after 3 and 6.5 years according to a standardized protocol.
Interviews: With participants after the first and second ultrasound examination, and with primary care physicians between baseline and the 3-year follow-up.
Stored samples of blood to the Medical Biobank: This was done at the baseline VIP visit and at 3- and 6.5-year follow-up visits among participants living in Umeå, to be used for analyses of novel biomarkers
Register data: Data from VIP-participation at baseline, Prescriptions, visits and risk factor measurements from the Medical records system in Region Västerbotten.
The Prescribed drugs, Hospitalizations and Causes of deaths registers at the National Board of Health and Welfare, results from the physical and psychological testing at age 18 years from the Conscripts Registry (male only), education and income from Statistics Sweden and air-borne pollutants from the Swedish Meteorological and Hydrological Institute.
Ten years after baseline: Morbidity due to CVD and revascularizing procedures and Causes of deaths, total and due to CVD, from registers at the National Board of Health and Welfare.
VIPVIZA has a separate database with access only by the database manager. The active researchers in the project has access to a digital dataportal that includes information about data and metadata information and a digital system for their proposals of new sub-projects and application for data, as well as a digital system for management of proposals by VIPVIZA:s steering group. Data is sent to researchers only after approval by the steering group and only depersonalized data, i.e. the social security number is replaced by a code and only the database manages has access to the code-key.
For further information, contact PI Ulf Näslund: ulf.naslund@umu.se
VPVIZA has a post at the Swedish National Dataservice https://snd.gu.se/en/catalogue/search/VIPVIZA
Acronym VIPVIZA
VIsualiZation of asymptomatic Atherisclerotic disease for optimum cardiovascular prevention – a pragmatic randomized controlled trial nested in Västerbotten Intervention Program. With VIP placed first in the acronym, it is marked that the project is carried out integrated in Västerbotten Intervention Programme (VIP).
1. Vanoli D, Lindqvist P, Wiklund U, Henein M, Näslund U. Fully automated on-screen carotid intima-media thickness measurement: a screening tool for subclinical atherosclerosis. J Clin Ultrasound 2013;41:333-9. https://doi.org/10.1002/jcu.22041
2. Vanoli D, Wiklund U, Lindqvist P, Henein M, Näslund U. Successful novice´s training in obtaining accurate assessment of carotid IMT using an automated ultrasound system. Eur Heart J Cardiovasc Imaging 2014;15:637-42. https://doi.org/10.1093/ehjci/jet254
3. Nyman E, Lindqvist P, Näslund U, Grönlund C. Risk marker variability in subclinical carotid plaques based on ultrasound are influenced by cardiac phase, echogenicity and size. Ultrasound Med Biol 2018;44:1742-50. https://doi.org/10.1016/j.ultrasmedbio.2018.03.013
4. Näslund U, Nawi N, Lundgren A, Fhärm E, Grönlund C, Johansson H, Lindahl B, Lindahl B, Lindvall K, Nilsson SK, Nordin M, Nordin S, Nyman E, Rocklöv J, Vanoli D, Wennberg P, Weinehall L, Wester P, Norberg M, for the VIPVIZA trial group. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA)– a pragmatic, open-label, randomised controlled trial. Lancet, Published online December 3, 2018 http://dx.doi.org/10.1016/S0140-6736(18)32818-6 – Lancet 2019;393:133-42. Erratum Lancet June 5 2019;393(10189):2394.
5. Näslund U, Ng N, Wennberg P, Norberg M. Patient-doctor engagement in cardiovascular prevention – Authors' reply. Lancet 2019 Aug 24;394(10199):e27. doi: 10.1016/S0140-6736(19)31335-2. PMID:31448748
6. Näslund U, Lundgren A, Vanoli D, Norberg M. Is intima-media thickness a predictor for cardiovascular risk? – Authors' reply. Lancet. 2019 Aug 3;394(10196):381. doi: 10.1016/S0140-6736(19)30343-5. PMID:31379329
7. Lindahl B, Nordin S, Nordin M, Johansson H, Lindvall K, Vanoli D, Ng N, Näslund U, Norberg M, Schulz P. Health literacy is independently associated with carotid atherosclerotic plaque and cardiovascular risk. Eur J Prev Card 2020;27:209-15, published on-line 2019 Oct 15 https://doi.org/10.1177/2047487319882821
8. Nyman E, Näslund U, Grönlund C. Inter-sonographer reproducibility of carotid ultrasound plaque detection using Mannheim consensus in subclinical atherosclerosis. Clin Physiol Funct Imaging 2020;40:46-51 https://doi.org/10.1111/cpf.12602
9. Bengtsson A, Norberg M, Ng N, Wester P, Carlberg B, Grönlund C, Hultdin J, Lindahl B, Lindahl B, Nordin S, Nyman E, Wennberg P, Näslund U for the VIPVIZA trial group. The beneficial effect over three years by pictorial information to patients and their physician about subclinical atherosclerosis and cardiovascular risk: Results from the VIPVIZA clinical trial. Am J Prev Card 2021 https://doi.org/10.1016/j.ajpc.2021.100199
10. Bengtsson A, Lindvall K, Norberg M, Fharm E. Increased knowledge makes a difference! - general practitioners' experiences of pictorial information about subclinical atherosclerosis for primary prevention: an interview study from the VIPVIZA trial. Scand J Prim Health Care 2021:1-8.
https://doi.org/10.1080/02813432.2021.1882083
11. Sjölander M, Carlberg B, Norberg M, Näslund U, Ng N. Prescription of lipid-lowering and antihypertensive drugs following pictorial information about subclinical atherosclerosis. A randomised controlled study. JAMA Network Open 2021;4(8):e2121683. https://pubmed.ncbi.nlm.nih.gov/34410393/
12. Schulz P, Lindahl B, Hartung U, Näslund U, Norberg M, Nordin S. The Right Pick: Does a Self-assessment Measurement Tool Correctly Identify Health Care Consumers with Inadequate Health Literacy? Patient Educ Couns. 2021 Jul 29:S0738-3991(21)00505-X. https://doi.org/10.1016/j.pec.2021.07.045
13. Kovrov O*, Landfors F*, Saar-Kovrov V, Naslund U, Olivecrona G. Lipoprotein size is a main determinant for the rate of hydrolysis by exogenous LPL in human plasma. J Lipid Res 2022;63:100144
https://doi:10.1016/j.jlr.2021.100144 * shared first authorship
14. Holmberg H, Sjölander M, Glader E-L, Näslund U, Carlberg C, Själander A. Time to initiation of lipid-lowering drugs for subclinical atherosclerosis. Sub study of VIPVIZA randomized controlled trial, with single arm cross-over. European Heart Journal Open 2022; 00:1-6. https://doi.org/10.1093/ehjopen/oeac003
15. Sommar JN, Norberg M, Gronlund C, Segersson D, Naslund U, Forsberg B. Long-term exposure to particulate air pollution and presence and progression of carotid artery plaques - A northern Sweden VIPVIZA cohort study. Environmental research 2022:113061. https://doi.org/10.1016/j.envres.2022.113061
16. Rohlen R, Jiang B, Nyman E, Wester P, Näslund U, Grönlund C. Inter-frame echo intensity variation of subregions and whole plaque in 2-D carotid ultrasonography – Simulations and in-vivo observations. J Ultrasound in Medicine 2022 Oct 20 http://doi.org/10.1002/jum.16114
17. Nyman E, Liv P, Wester P, Naslund U, Gronlund C. Carotid wall echogenicity at baseline associates with accelerated vascular aging in a middle-aged population. The international journal of cardiovascular imaging 2023. https://doi.org/10.1007/s10554-022-02760-3
18. Nyman E, Gronlund C, Vanoli D et al. Reduced progression of carotid intima media thickness by personalised pictorial presentation of subclinical atherosclerosis in VIPVIZA-A randomised controlled trial. Clin Physiol Funct Imaging 2023. https://doi.org/10.1111/cpf.12811
19. Andersson EM, Johansson H, Nordin S, Lindvall K. Cognitive and emotional reactions to pictorial-based risk communication on subclinical atherosclerosis: a qualitative study within the VIPVIZA trial. Scand J Prim Health Care 2023:1-12. https://doi.org/10.1080/02813432.2023.2178850
20. Näslund U, Norberg M, Wennberg P. The TANSNIP-PESA trial is not the end of the story. Eur Heart J 2023. https://doi.org/10.1093/eurheartj/ehad135
21. Fortuin-de Smidt M, Bergman F, Gronlund C et al. Early adulthood exercise capacity, but not muscle strength, associates with subclinical atherosclerosis 40 years later in Swedish men. Eur J Prev Cardiol 2023;30:407-415. https://doi.org/10.1093/eurjpc/zwad007
22. Ali H, Näslund U, Nyman E, Grönlund C. Translation of atherosclerotic disease features onto healthy carotid ultrasound images using domain-to-domain translation. Biomedical Signal Processing & Control 2023. https://www.sciencedirect.com/science/article/pii/S1746809423003191
23. Bengtsson A, Nyman E, Grönlund C, Wester P, Näslund U, Fhärm E, Norberg M. Multi-view carotid ultrasound is stronger associated with cardiovascular risk factors than presence of plaque or single carotid intima media thickness measurements in subclinical atherosclerosis. International Journal of Cardiovascular Imaging. https://doi.org/10.1007/s10554-023-02868-0
24. Nordin S, Braf I, Vallström C, Lindahl B, Nyman E, Näslund U, Norberg M. Associations between emotional support and cardiovascular risk factors and subclinical atherosclerosis in middle-age. Psychology & Health https://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-217344
25. Dante Salvador Jr, Per Liv, Margereta Norberg, Aurélie Pahud de Mortanges, Hugo Saner, Marija Glisic, Rachel Nicholl, Taulant Muka, Arjola Bano, Ulf Näslund. Changes in fasting plasma glucose and subclinical atherosclerosis: a cohort study from VIPVIZA trial. Atherosclerosis https://doi.org/10.1016/j.atherosclerosis.2023.117326
26. Andersson EM, Liv P, Nordin S, Näslund U, Lindvall K. Does a multi-component intervention including pictorial risk communication about subclinical atherosclerosis improve perceptions of cardiovascular disease risk without deteriorating efficacy beliefs? Social Science and Medicine 2023 https://www.sciencedirect.com/science/article/pii/S0277953623008870
27. Andersson EM, Lindvall K, Wennberg P, Johansson H, Nordin S. From risk communication about asymptomatic atherosclerosis to cognitive and emotional reactions and lifestyle modification. BMC Psychology 2024. https://doi.org/10.1186/s40359-023-01467-x
28. Holmberg H, Glader EL, Näslund U, Carlberg B, Sönnerstam E, Norberg M, Själander A. Improved adherence to statin treatment and differences in results between men and women after pictorial risk communication—a sub-study of the VIPVIZA RCT. European Journal of Clinical Pharmacology 2024. https://doi.org/10.1007/s00228-024-03694-6
29. Mickelsson M, Ekblom K, Stefansson K, Liv P, Nyman E, Själander A, Näslund U, Hultdin J. ABO Blood Groups, RhD Factor and Their Association with Subclinical Atherosclerosis Assessed by Carotid
Ultrasonography. Journal of Clinical Medicine 2024. https://doi.org/10.3390/jcm13051333
30. Mickelsson M, Ekblom K, Stefansson K, Liv P, Själander A, Näslund U, Hultdin J. ABO and RhD blood groups as contributors to dyslipidaemia – a cross‑sectional study. Lipids in Health and Disease 2025. https://doi.org/10.1186/s12944-025-02444-6
31. Mickelsson M, Ekblom K, Stefansson K, Själander A, Näslund U, Hultdin J. Exploring the extent of post-analytical errors, with a focus on transcription errors – an intervention within the VIPVIZA study. De Gruyter 2025. https://doi.org/10.1515/cclm-2025-0009
32. Norberg M, Liv P, Näslund U, Wester P, Andersson EM, Nordin S. The Path for Men from Young Adulthood Results of Cognitive Tests to Subclinical Atherosclerosis at Age 60: The Mediating Role of Socioeconomic Status, Lifestyle and Cardiovascular Disease Risk Factors – Results from a VIPVIZA Study. Rev Cardiovasc Med 2025; 26(3): 26312 https://doi.org/10.31083/RCM26312
33. Dahlin Almevall A, Wennberg P, Liv P, Nyman E, Lindvall K, Norberg M, Chorell E, Wennberg M. Midlife Mediterranean diet is associated with subclinical carotid atherosclerosis in late midlife. European Journal of Preventive Cardiology 2025. https://doi.org/10.1093/eurjpc/zwaf155