
Image: Johnér bildbyrå AB, Cultura Creative

Image: Johnér bildbyrå AB, Cultura Creative
The flowchart provides an overview of the work process when extracting health data and/or biobank samples. From the idea stage to the publication of results.
Here you will find information about the application process, ethical application, study planning, preparation of agreements and other important documents. It is a good idea to contact the Biobank Research Unit (EBF) at an early stage, preferably already at the idea stage.
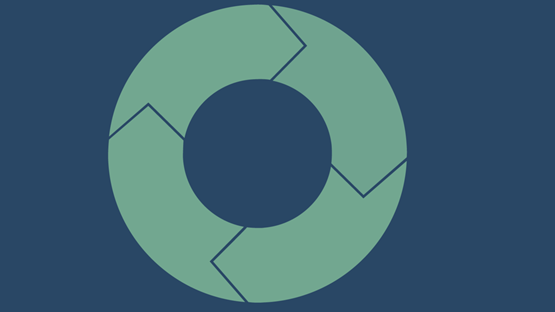
Flow chart Shows the work process from order to delivery.
What is NSHDS?
The Northern Sweden Health and Disease Study (NSHDS) is a collective designation for three population-based prospective cohort studies that collect survey data and associated blood samples: the Västerbotten Intervention Programme (VIP); the Northern Sweden MONItoring of trends and determinants in CArdiovascular disease (the MONICA study in Northern Sweden); and the Mammograph Screening Project. All three cohorts are harmonised in terms of samples. A large part of the data in VIP and the MONICA are also harmonised.
What types of samples and data are collected in NSHDS?
The three sub-cohorts in NHSDS have combined 140 000 participants, 129 000 of whom have provided blood samples. Half of the 129 000 have provided blood samples more than once. The sample collection mainly consists of EDTA and heparin blood samples separated into plasma, erythrocytes and buffy coat. DNA has been extracted from some of the material. Associated survey data on health-related lifestyle factors are available. Statistics on the number of cases for a given disease in NSHDS are also available. It is also possible to link NSHDS to other registers.
What is the Northern Sweden Diet Database (NSDD) and what data does it contain?
Survey data on dietary habits obtained from VIP and the MONICA study in Northern Sweden have been compiled in the Northern Sweden Diet Database (NSDD). The database also contains calculated nutrition data. Anna Winkvist is principle investigator for NSDD. An application form for accessing data from NSDD is available here.
What is PREDICT and what data and samples are available there?
PREDICT is a research infrastructure designed to increase the use of biobank samples in combination with health data to develop precision health and personalised medicine. The goal is to identify and quality-assure biomarkers that can be used by healthcare providers to prevent, treat or cure various diseases. PREDICT aims to create a research database from biobank samples. A large part of NSHDS – some 79,000 blood samples from 50,274 individuals – will be characterised using multi-omics and combined with survey and phenotype data.
Case-control data will be further enriched by integrating data from several mature cohorts with long follow-up periods, for example: diagnostic imaging; blood and tissue samples from cancer patients participating in U-CAN project; phenotype data from the Swedish CArdioPulmonary bioImage Study (SCAPIS), including diagnostic imaging; data from the Betula Project on aging, memory and dementia; and cardiovascular screening and ultrasound examinations from the project VIsualiZation of asymptomatic Atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA).
By promoting collaboration and the reuse of data, PREDICT strives to optimise the use of resources and make savings in valuable assets like blood samples, money and time. PREDICT involves a network of over 35 leading researchers working in more than 25 fields. You can learn more about PREDICT here.
What other samples and datasets are available?
The Biobank Research Unit at Umeå University coordinates the withdrawal of register data and samples from some 50 sample collections and registers. These include:
Who can apply for access to data and samples from NSHDS?
Researchers are welcome to apply for access to data and/or samples. Expert groups at the Biobank Research Unit assess the scientific value of all applications for access to sample and data collections coordinated within NSHDS. Among other criteria, expert groups assess research questions, study design, the availability of blood samples etc., and whether the applicant (or their supervisor or equivalent) has the requisite previous experience to suggest that the project is feasible.
In the case of applications from outside Umeå, the expert group may propose the participation of a local researcher who can contribute with knowledge and expertise concerning the study material.
How do I go about accessing data and/or samples from NSHDS?
We recommend that researchers contact the Biobank Research Unit early in the planning of their project, preferably at the concept stage. The Biobank Research Unit will be happy to offer advice on matters such as study design and ethical approval. Please contact the Biobank Research Unit (ebf@umu.se) to discuss the design of your study before submitting an application.
You will find an application form for accessing data and/or samples administered by the Biobank Research Unit here, along with a guide to the application process. The application should be sent to ebf@umu.se together with a copy of the research plan and a justification for the desired access. If possible, applicants should also submit variable lists (see point 3 here). It is also possible to submit variable lists later in the application process.
A separate form for access to the Northern Sweden Diet Database (NSDD) can be found here.
Who should I contact if I have any questions about the application process?
Interested researchers are asked to contact the Biobank Research Unit at ebf@umu.se early in the application process, preferably at the concept stage of their project. The Biobank Research Unit will be happy to offer advice on matters such as study design and ethical approval.
Expert groups are also happy to discuss new studies at an early stage. It is therefore a good idea to make early contact with the chairperson of the expert group, and even to submit a sketch for discussion. The expert group can then give feedback prior to a formal application. This will also allow the expert group to identify and inform the researcher of any potential synergies or competition with other studies. Contact details:
Who decides whether to grant access?
When your application is received, the Biobank Research Unit will contact the function that is responsible for approving your study. The scientific value of your research project will usually be assessed by one of the unit’s two expert groups, the Expert Group for Cancer or Expert Group for Cardiovascular and Other Diseases. There are also other groups whose decisions are reported in the expert groups:
How long will it take to process my application?
Applications and sketches received three weeks before an expert group meeting will be dealt with at the meeting. Applications received later will be dealt with time permitting. The dates of expert group meetings during the semester are regularly updated here.
Extracts from the expert group meeting minutes are usually sent to applicants two to three weeks after the meeting has been held. It may be necessary to supplement an application. If your application is approved, the Biobank Research Unit will attach practical information on matters such as fees, reporting personal data processing, risk and vulnerability analysis, a link to Umeå University’s checklist prior to starting a research project, as well as the name of the project and data administrators appointed by the unit.
How much does it cost to access data and/or samples from NSHDS?
You can find information on fees for withdrawing data and/or sample in NSHDS here. Note that we do not currently charge fees for administrative support and withdrawals in other bank deposit boxes/sample collections. Likewise, we have no fees for reviewing ethical applications and/or support in drafting up agreements in biobank studies.
What type of research support does the Biobank Research Unit offer?
The Biobank Research Unit coordinates the withdrawal of data and samples from some 50 sample collections and registers. We can offer advice on study design and collaboration. Our staff match cases and controls to studies, compile and harmonise data, and help with administrative formalities. Administrative support is also provided for other (non-NSHDS) biobank related research projects at Umeå University. The service is available to both Swedish and foreign researchers. The practical work is mainly performed by our statisticians and project administrators.
We offer advice and concrete assistance with:
Do I need ethical approval before I can apply for access to data/samples?
No. Ethical approval need not have been granted before you apply for access to data and/or samples. However, you must have ethical approval before a biobank agreement can be entered into and before preparations for data and/or sample withdrawal begin. Please refer to our workflow chart posted above the FAQs.
We recommend that researchers ask the Biobank Research Unit’s for assistance when writing an application for ethical approval. These days, there are many details that need to be addressed in applications, such as access to register data, sample withdrawals and personal data processing. The Biobank Research Unit will provide you with a template for an ethical application that includes suitable formulations, particularly with regard to data processing. We also recommend the Biobank Research Unit reviews your ethical application before it is submitted to the Swedish Ethical Review Authority.
What type of agreements are required to access data and/or samples?
If your study involves human biological material, you will need to conclude a biobank agreement with Biobank North at Region Västerbotten. You will find forms to access samples (L1.1) and to send samples for analysis outside Region Västerbotten (L2a1/L2a3) on the Biobank Sweden website. The Biobank Research Unit will help you to choose the correct forms and help draft the agreement. The Biobank Research Unit mediates the commuication between researchers and the Biobank North regarding NSDHS studies and other projects for which Umeå University is the entity responsible for research.
If your study involves partners and/or labs outside Umeå University, third-party agreements between Umeå University and the recipients of samples and/or data must also be concluded. The Biobank Research Unit works with the University’s legal officers to support researchers in drawing up third-party agreements. The legal officers provide the Biobank Research Unit with the latest versions of templates for agreements with a collaborator (Umu-MTA with collaboration agreement) or processing laboratory (Umu-MTA and a Personal Data Processing Agreement (DPA)). If the processing lab is a company, a service agreement is often concluded and attached to the MTA and DPA. The company is responsible for drawing up the service agreement in consultation with the researcher. If samples and data are transferred outside the EU/EEA, a data protection impact assessment must be performed and agreement drawn up with the EU Standard Contractual Clauses (SCC). The Biobank Research Unit will assist in the preparation of agreements and mediate the communication between researchers and the University’s legal officer.
Who prepares these agreements and who is required to sign them?
The Biobank Research Unit always has access to the latest agreement templates and based on these prepares agreements for all NSHDS studies as well as other biobank related studies for which Umeå University is the entity responsible for research. See workflow chart posted above the FAQs. These include the following:
Agreements with region Västerbotten:
Third-party agreements:
What is the difference between the Biobank Research Unit and Biobank North?
The Biobank Research Unit at Umeå University and Biobank North at Region Västerbotten work closely together.
The Biobank Research Unit is part of the Faculty of Medicine at Umeå University. The current director is Ingvar Bergdahl. The Biobank Research Unit coordinates the withdrawal of data and samples from some 50 sample collections and registers. We can offer advice on study design and collaboration. Our staff match cases and controls to studies, compile and harmonise data, and assist with applications for ethical approval and certain legal agreements related to biobank related research projects at Umeå University. The service is available to both Swedish and foreign researchers.
Biobank North is a division of Laboratory Medicine Västerbotten. Biobank North is responsible for most clinical and research sample collections within Region Västerbotten. As the responsible authority for register data and biological material, Region Västerbotten is responsible for ensuring compliance with and the application of the Swedish Biobank Act, and ultimately for deciding who has access to samples for research purposes. Biobank North provides operative services throughout the sample collection process, from study integration via sample handling and storage to the withdrawal of samples. This includes offering advice and support on practical and logistical matters such as handling, labelling and refrigerating samples prior to a new research projects and new collections of biobank samples.
Who manages sample withdrawals?
Biobank North is responsible for all sample handling during the collection and withdrawal of samples. Withdrawals of samples for NSHDS projects are planned together with the Biobank Research Unit. See workflow chart above.
The Biobank Research Unit identifies suitable blood samples based on the desired study design, usually by linking NSHDS to other disease registers, quality registers and other specified registers. Controls are randomly selected from the entire NSHDS cohort by matching, for example, the dates of blood sampling with corresponding disease cases. After identifying samples for analysis, the Biobank Research Unit sends an order to Biobank North, which arranges sample withdrawal. Samples are prepared based on the researcher’s and receiving laboratory’s requirements concerning type of tube, sample handling, etc. Pseudonymised (coded) samples are then sent for analysis according to the researcher’s instructions.
How long does it take to access biological material and who should I contact with inquiries?
It is difficult to say how long it will take from when you apply until you have access to samples. In the case of a completely new project, it will usually take 1-2 years, but it can be much quicker to extend an existing study. A general indication of the time required for each step is shown in the above workflow chart.
Once an application is approved by the expert group, the researcher is recommended to contact the Biobank Research Unit as soon as possible for the project to be activated. A project and data administrator will then be assigned, and the structure of the project can be clarified in greater detail. It will then be possible to estimate a timeline, which in turn will depend on the existing queue for samples, which agreements need to be drawn up and so on. During the planning stage, the researcher should also contact the laboratory it plans to use to analyse the samples. Some companies draw up a service contract, in which case the Biobank Research Unit should be informed so that a copy can be attached to the third-party agreement.
Contact with Biobank North will go through the project administrators at the Biobank Research Unit. The Biobank Research Unit and Biobank North jointly plan the withdrawal of samples. Drawing up agreements may cause a bottleneck in the process, especially if samples/data are to be transferred outside the EU/EEA. It is recommended that the researcher contacts the Biobank Research Unit in good time so that work on drawing up agreements can begin at an early stage. Once a biobank agreement is in place, Biobank North will get in touch with the contact person designated in the application to plan how and where samples are to be dispatched. The handling time at the lab depends on both the scope of the study (number of samples) and how many other studies are in the queue. During 2022, in most cases the handling time for drawing up agreements to access samples has exceeded six months.
Who manages data withdrawals?
When a project is activated at the Biobank Research Unit, one or more administrators will be assigned to deal with data processing. The administrator will then open a dialogue with the researcher/applicant. A database will then be compiled based on the required registers withdrawals and NSHDS variable lists.
Survey data and dietary data is generated automatically based on the completed withdrawal forms using a data script that can be modified depending on the study design. Depending on how the project is structured, additional data harmonisation may be required. Administrators handling the withdrawal will use programming to ensure extraction of the correct samples and data. The Biobank Research Unit will prepare and pseudonymise data.
The Biobank Research Unit can also assist in withdrawals from other registers, such as the Total Population Register, National Patient Register, Prescribed Drug Register, Cause-of-Death Register, National Diabetes Register, National Cancer Register, regional cancer registers and others. Archived register data at Umeå University may be reused but only after the study in question has received ethical approval.
Please note that you must have ethical approval before any data can be withdrawn. The ethical approval must contain a detailed description of the research data procedure including which data will be obtained from which registers and whether it will be sent with personal identifiers (i.e., the Swedish personal number). The ethical approval should also state whether archived register data at Umeå University will be re-used.
How do I identify cases?
In some studies, cases are already defined, while in others they must be identified based on disease registers. A application will then be submitted to the register holder (e.g., Statistics Sweden, the National Board of Health and Welfare or Region Västerbotten) to request a data withdrawal from case registers. To withdraw data, the applicant must have ethical approval and the register holder must conduct an examination of detriment and secrecy. Data is provided to the entity responsible for research, i.e., Umeå university. Usually we apply for the data to be sent to the Biobank Research Unit who then compiles a pseudonymised dataset for the researcher. If necessary, the research group in question may perform further verification of cases by reviewing medical records.
How are cases and controls matched?
In the case of blood sample studies, data administrators begin with an extract from the laboratory information management system (LIMS) to ascertain sample stock status for the relevant cohort from which controls can then be matched and randomised. For certain studies it may be necessary to reduce the eligible control cohort in various ways, such as by excluding everyone with cancer. Building up a study often involves data from many different types of registers held by different authorities (in addition to health data registers and sample data in LIMS). There may also be many pre-diagnosis blood samples that need to be prioritised based on given criteria. Or the project may involve repeated sampling, leading to a complex study design.
A research study protocol is filled in for studies with a complex design. The protocol states details such as selection of cases and exclusion and matching criteria for controls/cohorts. This document should also clarify details concerning the samples.
EXAMPLE CRITERIA FOR MATCHING
Selection criteria:
1. Same cohort (VIP, MONICA, MAMAR)
2. Same sex
3. Date of sampling ± 1 month
4. Age ± 1 year
5. Same smoker status
6. Simple random sampling
7. The control must not have had cancer before the index date (date of diagnosis)
8. Alive on the date the case was diagnosed
Relax 1: Sample date ± 3 months; Age ± 1 year
Relax 2: Sample date ± 6 months; Age ± 5 year
How is data processed in accordance with GDPR?
Personal data such as samples, analytical data and other information provided by participants is stored in data registers at the University Hospital of Umeå and Umeå University. These registers are used to identify blood samples for new research studies through coprocessing with disease registers, national quality registers and other registers. The Biobank Research Unit sends the criteria for sample selection to Biobank North, which then prepares and dispatches coded samples for analysis according to the researcher’s requirements. So, samples are only identifiable by a code number and cannot be linked to an identified person without access to the code key.
As the Biobank Research Unit has access to personal identity numbers, it can also compile other data requested for the study. The unit has been dealing with such requests since the 1990s and has secure procedures for working with sensitive personal data. All of the unit’s work – including documentation, data, and key-codes – is stored on secure servers with two-factor authentication at IT Support and System Development (ITS) at Umeå University.
The Biobank Research Unit does not disclose personal identity numbers to researchers, who only receive pseudonymised samples and/or health data. Certain studies do however require medical record reviews or similar measures that involve the processing of personal identity numbers, but only once ethical approval has been granted. Once the final dataset is compiled, before it is delivered to the researcher the data is pseudonymised (key-coded) by replacing the personal identity number with a unique Study ID. The pseudonymised database is password protected and only processed by members of the responsible research group and/or any collaborators in the study. The key is retained by the Biobank Research Unit for quality assurance and monitoring purposes or future withdrawals of follow-up data. Pursuant to archiving rules, all released registers are kept at the Biobank Research Unit. Registers can only be reused for new studies after ethical approval is renewed.
Many research studies involve collaboration with an analytical laboratory in Sweden, another EU/EEA country or third country. In such cases, a data processing agreement is always drawn up to regulate how personal data is processed during and after the project. If personal data is transferred to a third country (a country outside the EU/EEA) or an intergovernmental organisation, Umeå University takes all reasonable legal, organisational, and technical measures required to ensure an adequate level of data protection.
You can learn more about how personal data is processed on the University’s website under Information for study participants.
How is data sent in accordance with GDPR?
Pursuant to Umeå University’s information security policy, the Biobank Research Unit uses the University’s encryption service Skyddad Bilaga [Protected Attachment] to deliver pseudonymised data to the principal investigator. A data delivery acknowledgement form is signed by the recipient and returned to the Biobank Research Unit. Skyddad Bilaga is a service that employees of Umeå University can use to transfer SSL-encrypted files.
How can researchers process/store data securely in accordance with GDPR?
The principal investigator and their head of department bear the ultimate responsibility for ensuring compliance with Umeå University’s information security regulations. The principal investigator is informed of the regulations in a risk and vulnerability analysis (RVA) template supplied after their study has been approved by one of the unit’s expert groups.
Once laboratory analysis is complete, the Biobank Research Unit will compile the lab results with clinical and lifestyle data, meaning that as a rule the principal investigator will not need access personal identifiers and/or any code keys. All staff involved in the project must comply with the University’s information security regulations and data must be stored in a secure manner that prevents unauthorised access. Read the checklist prior to starting a research project (umu.se).
How are study participants informed about personal data processing?
GDPR states that the controller shall take appropriate measures to protect the data subject’s rights and freedoms and legitimate interests, including making information about the processing of personal data publicly available. One way for researchers to fulfil this requirement is to publish a page on the Faculty of Medicine’s research projects page.
A template for project pages is sent out with the extract from the expert group meeting minutes after project approval. We recommend linking to the Biobank Research Unit’s page with information to participants in studies under the project description (a link is already included in the aforementioned template). You can find further information about the content of project pages here.
What general regulations apply to the withdrawal of samples and data?
The following general regulations apply:
Who is responsible for reporting personal data processing?
The principal investigator is responsible for reporting that personal data is to be processed within the scope of the research project, with the exception of projects within the European Prospective Investigation into Diet and Cancer (EPIC), where the Biobank Research Unit will assist with the reporting of personal data processing. Learn more on the staff website under personal data processing in research and reporting personal data processing.
To register your personal data processing in Umeå University’s register, follow the link to the registration form: https://portal.diarie.umu.se/anmalan-om-personuppgiftsbehandling
As you cannot save the form while you are filling it in, you should read the form first to ensure you have all relevant information at hand. It is recommended that you use the Chrome browser, as this is the most compatible with the form.
If you have any questions about reporting personal data processing, please address them to pulo@umu.se.
What is a risk and vulnerability analysis (RVA) and who is responsible for performing one?
According to procedure at Umeå University, all researchers granted access to samples and/or data from NSHDS are required to perform an analysis of the risks and vulnerabilities associated with their project.
In collaboration with Umeå University’s information security coordinator and IT-specialist, the Biobank Research Unit has prepared a template for a simplified information classification and risk and vulnerability analysis (RVA). This template is sent out with the extract from the expert group meeting minutes after project approval. Completed RSAs must be archived at your department and a copy sent to the Biobank Research Unit.
What is a data protection impact assessment and who is responsible for performing one?
If you intend to transfer samples and/or data to a third country (outside the EU/EEA) and/or your project uses large amounts of sensitive personal data, an data protection impact assessment must be conducted pursuant to Article 35 of GDPR. This is intended to minimise the risks presented by the processing of personal data to the rights and freedoms of natural persons before they arise. Risks should primarily be assessed from a data protection and privacy perspective, but also on the basis of fundamental rights such as freedom of expression, thought, movement, conscience and religion and the prohibition of discrimination.
The impact assessment template is filled in by the researcher with the assistance of the Biobank Research Unit, then reviewed by the University’s data protection officer and/or legal officer. The completed information classification and risk and vulnerability analysis form the basis for the impact assessment, which in turn is used to draw up the SCC agreement. Once drawn up, the SCC agreement is reviewed by the University’s legal officer and then signed by University Director at Umeå University and an authorised signatory of the laboratory or company performing the analysis.
What rules apply to transferring data and/or samples to a third country?
If personal data and/or coded samples are to be sent to a third country – i.e., a country outside the EU/EEA or to an intergovernmental organisation – an agreement must be drawn up between Umeå University and the recipient containing the EU’s Standard Contractual Clauses, otherwise the transfer is unlawful. Learn more on the website of the Swedish Authority for Privacy Protection (IMY) at https://www.imy.en/. The Biobank Research Unit prepares the necessary agreements. It is not permitted to modify the Standard Contractual Clauses.
Where can I find methodology descriptions for the NSHDS cohorts?
You can find a document on the methods used in NSHDS under “Publications, method document and descriptive statistics” here.
Where can I find information about consent and information provided to NSHDS participants?
The consent forms have varied over time. A compilation of the consent forms that have been used when blood samples are taken for research are available here [LINK to a compilation of consent on the website].
What is the policy on publishing studies that have used NSHDS data/samples?
Guidelines for the publication of studies based on NSHDS are available here. The following acknowledgement should be included. The italicised text should be adapted to the study in question.
We thank (in consortia with other cohorts: NSHDS investigators thank) the Biobank Research Unit at Umeå University, Västerbotten Intervention Programme, MONICA study in Northern Sweden and Region Västerbotten for providing data and samples and acknowledge the contribution of Biobank Sweden, supported by the Swedish Research Council (VR 2017-00650).
The Biobank Research Unit compiles annual statistics on publications and validation studies that have used samples and/or data collected for NSHDS. It is therefore important to include the above acknowledgement to facilitate the compilation of statistics.
Must I have a coauthor from NSHDS or Umeå University?
No. But, as a researcher, you will often benefit from collaborating with other researchers with experience of NSHDS and/or who have collected and arranged the data you use. We apply ICMJE Recommendations on Defining the Role of Authors and Contributors. The ICMJE recommends that anyone making a substantial contribution to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work, should have the opportunity to participate in the review, drafting, and final approval of the manuscript, thus qualifying for authorship.
International studies require a Swedish principal investigator to take ethical responsibility for that part of the research to be conducted in Sweden. This will normally involve a significant scientific contribution.
What rules apply to archiving sample analysis data?
The terms and conditions of the application to withdraw data/samples include a requirement to send generated laboratory data to the Biobank Research Unit. Data will only be reused with the permission of the researcher who generated it. You can learn more here.
Researchers can register their processed datasets with the Swedish National Data Service (SND). Datasets including personal data cannot be registered, although the researcher may register metadata concerning the content of the dataset and their contact details.
Researchers should save processed datasets containing personal data on a secure server at the Biobank Research Unit. For further information, please email us at ebf@umu.se.
Common abbreviations
BBN
Biobank North, an organisational entity legally responsible for samples taken by healthcare providers pursuant to the Swedish Biobank Act (SFS 2023:38) and an operational unit of Region Västerbotten that deals with the collection, processing and withdrawal of biobank samples.
EBF
The Biobank Research Unit at Umeå University, coordinates the withdrawal of register data and samples from some 50 sample collections, including NSHDS.
NSHDS
The Northern Sweden Health and Disease Study, the collective designation for three population-based prospective cohort studies that collect survey data and associated samples:
NSDD
The Northern Sweden Diet Database of survey data concerning dietary habits obtained from VIP and MONICA.
PREDICT
Precision Medicine Data Collaboration, research infrastructure at Umeå University designed to promote the use of existing biobank samples for research into biomarkers and precision medicine. This will be achieved through the creation of a database of health data and blood sample analysis data (so-called multi-omics data). A subset of blood samples collected by VIP are included in PREDICT.
LIMS
Laboratory information management system, the biobank’s register of sample data, including information about the sample, participants consent and biobank agreements associated with the sample collection.
GDPR
The General Data Protection Regulation.
L1.1
A biobank agreement drawn up for each new sample collection. The agreement regulates the right of researchers to use the samples, as well as the responsibility of the principal of a biobank for traceability.
L2a1
An agreement to dispatch samples for use outside Region Västerbotten, including when samples will be analysed at Umeå University.
MTA
Material transfer agreement, drawn up when samples are transferred from Umeå University, often referred to as an Umu-MTA.
DPA
Data processing agreement, drawn up when data and/or samples are sent to another party (“processor”) to be processed on behalf of the controller.
RVA
Risk and vulnerability analysis, performed in conjunction with data classification at the start of a research project. Attached to the responsible researcher’s report of personal data processing and stored in Umeå University’s registry.
DPIA
Data Protection Impact assessment, performed to identify risks arising out of the processing of personal data and to minimise these risks as far and as early as possible, e.g. when large amounts of data/samples are to be transferred to a third country (a country outside the EU/EEA).
SCC
EU Standard Contractual Clauses. Must be included in all agreements drawn up for the transfer of personal data to a third country (outside the EU/EEA).
Umu
Umeå University
My question is not among the FAQs, what should I do?
Contact us at ebf@umu.se and we will be happy to help!